A technique for measuring ice nucleating aerosols (IN) with a continuous flow diffusion chamber is described. Airborne IN measurements were made on NASA's DC-8 Airborne Laboratory during the April-May 1996 SUCCESS project. Most sampling was done in the upper troposphere. Processing conditions ranged from about -15 to -40°C and from ice saturation to ~15% water supersaturation. Ambient IN concentrations were <0.1 to ~500 l-1, and were generally greater at colder temperatures and higher supersaturations. Within limited sample periods, the IN concentration related strongly to sampling temperature and supersaturation, but overall, there was wide variability. An equation describing the concentration of heterogeneous freezing nuclei was derived from a subset of the data and was used in a microphysical parcel model to simulate ice formation in cirrus conditions (-46°C and updrafts 10 to 50 cm s-1). Heterogeneous freezing nuclei formed the first ice in rising air parcels, with the consequence of decreasing the maximum ice crystal concentration in comparison with simulations having only homogeneous freezing. The decrease is as large as much as a factor 10. This suggests that ice nuclei expand the conditions in which cirrus clouds form and broaden the ice crystal size distributions.
Remote sensing observations and theoretical studies of the earth's climate show that clouds provide a strong influence on the radiative energy balance, but many of the factors that regulate the influence of clouds are not well understood. It is known, for example, that water clouds and ice clouds have substantially different microphysical and radiative properties. Ice nucleating aerosols (IN) have special significance in this context because the abundance and characteristics of IN affect the formation of ice crystals in clouds, thereby influencing the concentration, shape and phase of cloud particles, the size and persistence of clouds, precipitation efficiency and radiative exchange processes.
IN are a special subset of total aerosol, characterized only by their ability to nucleate the ice phase. Measuring the characteristics of ice nuclei is difficult because the activity depends on both temperature and humidity, there are multiple nucleation mechanisms, and there is no clear association with size or chemical properties of the IN aerosol
The goals of our recent research on ice nucleation are to estimate
the concentrations, spatial distributions, sizes and chemical
composition of IN and non-IN aerosols in background air and in
regions affected by aircraft exhaust. Measurements of IN were
obtained during a recent field project, SUCCESS (Subsonic Aircraft:
Contrail and Cloud Effects Special Study). A central question
of SUCCESS is to assess the effect that aircraft exhaust can have
on climate through direct effects (gases, contrails and aerosols)
and indirect effects on high altitude clouds.
There is potential for ice nuclei to affect clouds, weather and climate through the following key factors:
Descriptions of natural and manmade IN are in the realm of research material; they are not routinely available for applications involving weather analysis and forecasting. Future research may provide advanced capabilities to use such information through:
The characteristics of IN affect the microphysical properties of clouds, specifically, the concentration ice crystals and the balance between sources and sinks of water vapor. These are basic and important factors. In the context of different atmospheric conditions and dynamic forcing mechanisms, the microphysical properties determine the potential for forming precipitation, the evolution of precipitation, the persistence of clouds, and aspects of cloud radiative properties (e.g., Braham 1986). All of these have substantial importance for weather forecasting and for climate. For example, remote sensing observations and theoretical studies show that clouds provide a strong influence on the earth's radiative energy balance, but many of the factors that regulate the influence of clouds are not well understood. Ice formation is one of those factors.
IN aerosols are continuously and efficiently removed from the atmosphere by scavenging and precipitation. They are produced from a wide variety of natural and anthropogenic sources, but little, if anything, is known about these sources. Basic information to characterize IN production should ultimately include surveys of source locations, production rates, seasonality and connections with biological processes and industrial activity. But at the present time, that kind of information is not known.
This paper describes IN measurements with a newly developed continuous flow diffusion chamber instrument and presents simulations that use IN descriptions in a microphysical model.
All IN measurements are based on exposing aerosols to a cold supersaturated environment and counting the ice crystals that nucleate and grow to detectable size. All methods show that IN concentration increases in response to colder temperature. Methods that control supersaturation show that IN concentration increases strongly with higher supersaturation. A variety of measurement techniques have been developed, each with different advantages and disadvantages. We listed here the more popular methods:
The following section describes a CFD that we developed in the past few years. It has been used to obtain real-time measurements of IN from aircraft. It also has been used to isolate and collect IN aerosols to examine their size and composition. This is a research, prototype instrument. It may not be suitable for large scale or long term monitoring applications, but it is providing an opportunity for unique and basic studies of IN.
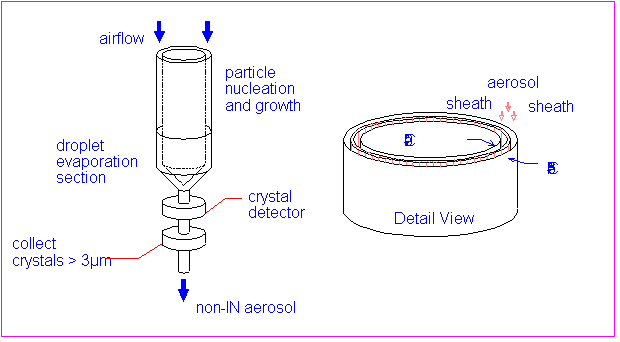
The CFD is a thermal gradient diffusion chamber of concentric
cylinder design (Rogers 1988; 1994). Fig. 1 shows the operating
principles. Air flows vertically downward in the annular space
between two ice-coated cylinders that are at different temperatures.
Sample air occupies the central 10% of the flow; it is sandwiched
between particle-free sheath flows and exposed to a supersaturation
determined by the temperature difference. Active IN aerosols form
ice crystals that grow to ~3µm diameter or larger, depending
on temperature, humidity and residence time. The droplet evaporation
section occupies the lower third of the chamber, where the warm
ice vapor source is removed, and the humidity drops to ice saturation.
Particles >3µm (ice crystals) are counted at the chamber
outlet by a Climet 7350A optical particle counter (OPC).

Fig. 1. Operating principles of CFD. Air flows between
ice-covered walls held at two different temperatures. Particles
nucleate and grow to detectable size in supersaturated region.
With walls -20 and -36°C, sample conditions are -30°C
and 1% supersaturation. Typical residence time ~3-5s.
In order to capture crystals (containing IN) on electron microscope grids, an inertial impactor was located immediately downstream of the OPC. Typically, several hundred crystals can be collected on a grid. Single particle analysis techniques are then used to determine the number, size, morphology and chemical composition of the nuclei (Kreidenweis et al. 1997).
During the SUCCESS project, IN sampling in the CFD covered a wide
range of conditions, from about 15 to -40°C and from
ice saturation to 20% water supersaturation (SSw). The range
is shown in Fig. 2, bounded on the left by the minimum SSw limit,
ice saturation, which is temperature dependent. The upper SSw
bound is calculated from wall temperatures, but modeling simulations
suggest that high concentrations of droplets ultimately limit
the actual SSw by serving as vapor sinks. Thus, the real maximum
limit is perhaps ~10-15% SSw. The CFD samples at only one temperature
and SSw at a time; changing the conditions (wall
temperatures) takes a few minutes. Two sampling approaches were
used: (1) constant temperature and SSw; and (2) varying temperature
or SSw. The second approach is an attempt to estimate the thermodynamic
response of IN (spectra), but the inherent limitation is the continuously
changing sample air. As operated during SUCCESS, the CFD had
no sensitivity to detect homogeneous freezing or contact-freezing.
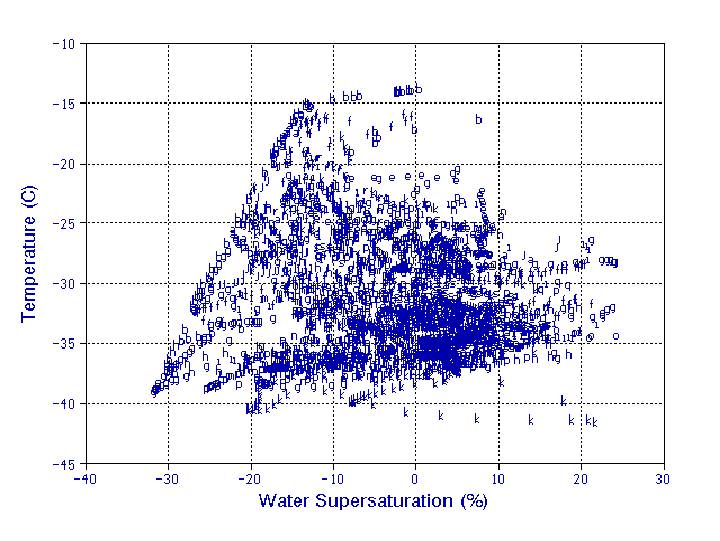
Fig. 2. Range of temperatures and supersaturation conditions in CFD for all ice nuclei measurements during 19 flights. Letters identify flight and represent one minute averages.
A large quantity of IN measurements were collected during SUCCESS.
The results shown here represent some aspects of the larger data
set and illustrate the type of data obtained. Overall, the IN
concentration ranged from the lower detection limit ~0.1 to over
100 l-1
as shown in Fig. 3. For this figure, all measurements
were first integrated into ~1 minute averages (~1 liter of air);
60% of the data fell between 2 and 20 l-1.
There were also many zeroes (i.e., below the sampling threshold)
and a few periods with very high concentration. An estimate of
the IN fraction of total aerosol was made from simultaneous measurements
of the total concentration of particles (diameter > 0.012µm
diameter); out of a million CN, there were only ~10 to 1000 IN.
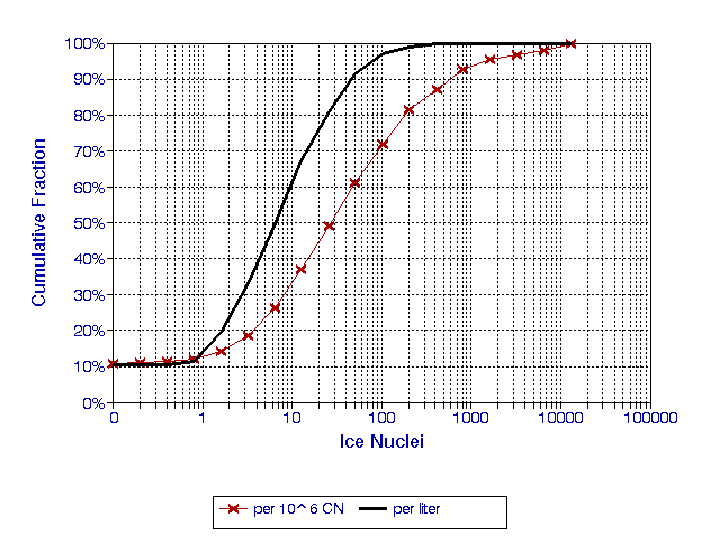
Fig. 3. Cumulative frequency distribution of IN concentrations
during SUCCESS, for all CFD temperatures and supersaturations.
Measurements were first integrated into ~1 minute samples (~1
liter). Also shown is ratio of IN to CN measured at the same
time.
Ice formation at the low temperatures in upper tropospheric clouds may result from both homogeneous freezing of solution droplets formed on soluble CCN aerosols and by heterogeneous nucleation of ice on insoluble or partially insoluble aerosols. Homogeneous freezing is thought to dominate at temperatures colder than -40C, but the role of heterogeneous nucleation has been open to speculation due to uncertainty about the concentrations and properties of suitable ice nuclei in the upper troposphere.
One of the methods of operating the CFD during SUCCESS involved
cycling sample humidity to determine the supersaturation spectra
of IN at a nearly constant temperature. An example of this operational
procedure is shown in Fig. 4 for an approximately 50 km flight
segment above cirrus clouds over Oklahoma. The same data are plotted
in Fig. 5 to show the strong response of IN to supersaturation
below 6%. Above a few percent supersaturation, however, the IN
concentration was relatively constant. This suggests that all
of the aerosols which acted as freezing nuclei at 33°C
were immersed in dilute liquid droplets at supersaturations above
a few percent.
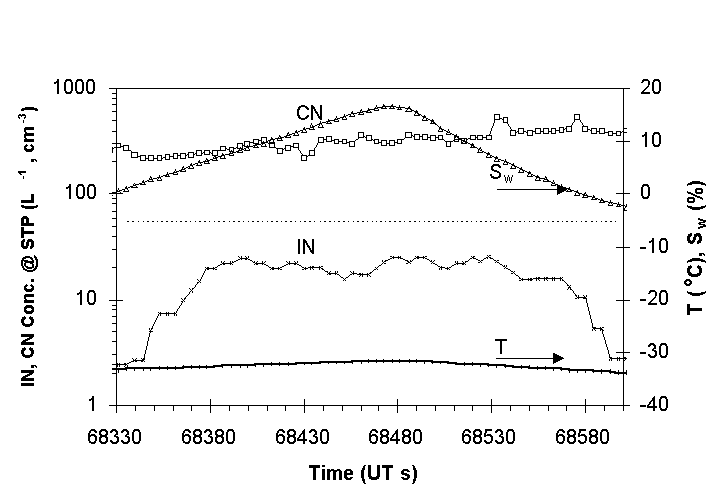
Fig. 4. Approximately 5 minute period showing IN
and CN (total particles with diameter >0.012µm) in the
upper troposphere on April 24, 1996, above a cirrus deck at 11.6
km, -61C. CFD temperature ~33°C; water vapor supersaturation
varied. Values are 4s averages.
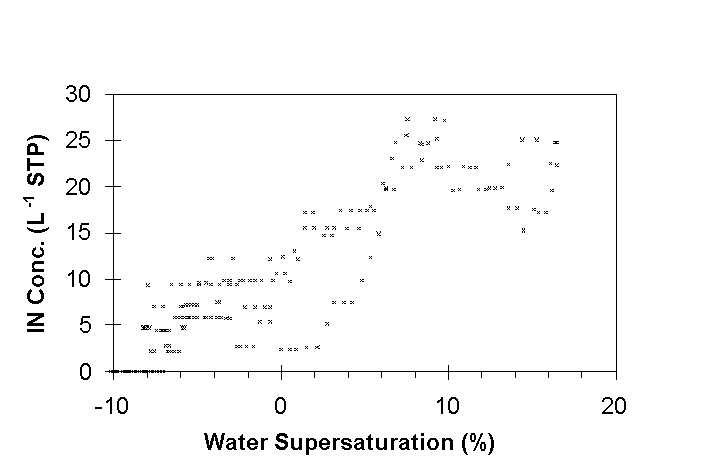
Fig. 5. IN concentrations from Fig. 4 plotted versus CFD water supersaturation.
Data of the type shown in Fig.5 were accumulated for a number
of level flight segments at altitudes between about 9 and 12 km
on three different days, from mostly clear air regions that did
not display any sharp transitions in CN concentrations or aerosol
concentrations in the 0.1 to 3m size range during a flight segment.
The value of water supersaturation assumed for the complete activation
of immersion freezing nuclei was selected as 3%, based on subjective
analysis. The freezing nucleus temperature spectrum determined
from these selected time periods is shown in Fig. 6, along with
a power law regression ( 1) for the data. IN concentrations ranged
from a low of 1 l-1
at 27°C to 100 l-1
at 35°C. These IN concentrations were normalized by
the concurrent CN concentrations because it improved the goodness
of fit (~ doubled r2). Although this improvement suggests
a general correlation of freezing nuclei with CN, this may only
be the case for the conditions selected. Namely, regions of recent
particle formation or depletion due to cloud processing were not
included. In other regions, where new particle formation or particle
depletion was evident, IN appeared to vary along with aerosol
concentrations above 0.1m. This observation is consistent with
a modal size of IN aerosols in the accumulation mode, as determined
from transmission electron microscopy analyses of the residues
of ice crystals from the CFD (Chen et al., 1997). It is the subject
of continuing analyses.
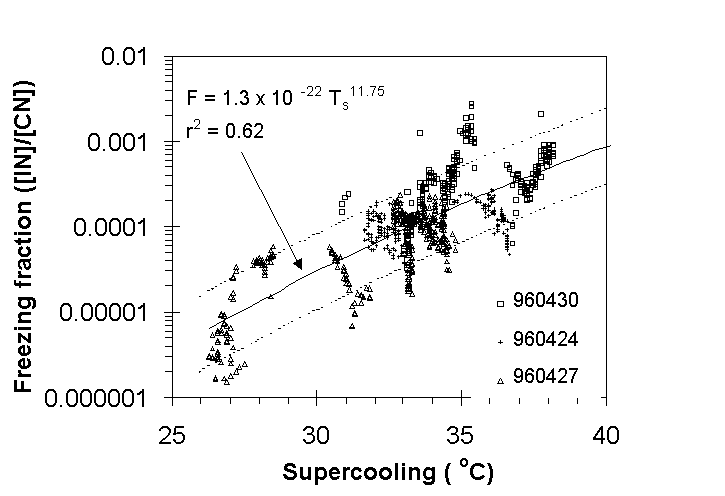
Fig 6. Freezing nuclei concentrations for SSw>3%,
normalized by [CN], as functions of supercooling below 0C for
segments of three DC-8 flights in the upper troposphere. Power
law fit (solid) and its 1 uncertainty (dashed).
The measured heterogeneous freezing nuclei spectrum was used in
the adiabatic parcel model described by DeMott et al. (1997a)
to investigate the role of heterogeneous IN in ice formation in
cirrus clouds. In this model, nucleation and growth of droplets
and ice crystals are calculated for an adiabatically-cooling,
non-precipitating parcel of air in an updraft with specified initial
temperature and humidity. Homogeneous freezing nucleation rates
are computed assuming pure sulfuric acid CCN, based on the parameterization
in DeMott et al. (1997a). Heterogeneous freezing nucleation was
simulated by assuming that these IN must also act as CCN and that
their soluble components act to depress the freezing points (DeMott
et al., 1997b). An uncertainty in applying the results is determining
what fraction of the IN that activated at high supersaturations
were also CCN. Energy dispersive x-ray analyses (EDS) of IN residues
during SUCCESS indicate that 3 to 27% of the IN contain elements
associated with soluble compounds and probably were CCN.
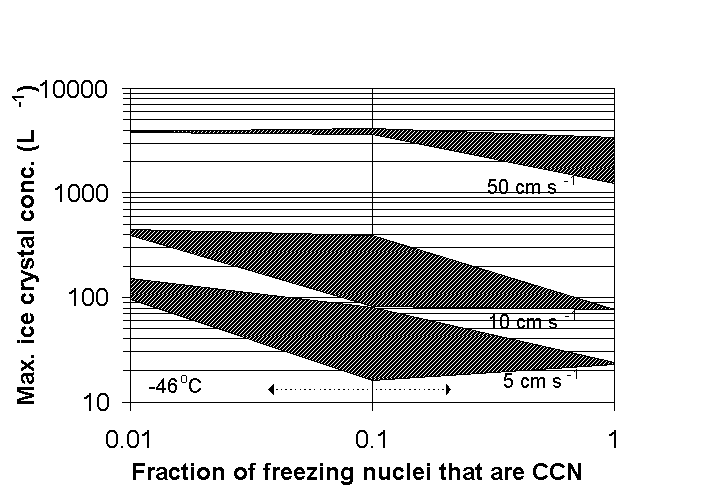
Fig 7. Model predictions of maximum crystal concentrations
in cirrus. Results from 18 simulations are shown. Air parcels
initialized at -46C. Shaded regions at three vertical velocities
bound the ice crystal concentrations formed when employing the
upper and lower uncertainties of the power law freezing nucleus
spectrum. The range of observed fractions of IN containing soluble
matter, based on TEM studies, is given by the arrow. Ice forms
by a combination of heterogeneous and homogeneous nucleation.
Homogeneous freezing dominates on the left side (few CCN contain
IN). Heterogeneous freezing dominates on the right (all CCN contain
IN).
Figure 7 summarizes these cirrus simulations. The existence of heterogeneous freezing nuclei decreases the maximum ice crystal concentration that forms, compared to homogeneous freezing alone. Ice crystal concentrations are reduced up to one order of magnitude. This is a large impact on cirrus properties, but the uncertainty in how many of the freezing nuclei are also active as CCN leads to a large uncertainty in their expected impact on cirrus ice formation. A typical value of 10% of IN being CCN, inferred from the chemical analyses, is within the region of maximum impact. In other simulations, not shown here, heterogeneous freezing nuclei had a negligible impact below -65C.
Measurements of ice formation in wave clouds during SUCCESS support the existence of the measured concentrations of heterogeneous ice nuclei in the upper troposphere and their role in forming the first ice in rising air parcels. The consequences of the presence of ice nuclei in upper tropospheric clouds are to expand the conditions over which the clouds will form and to broaden the ice crystal size distributions (not shown here).
This example demonstrates the application of IN measurements to improve numerical predictions of cirrus clouds and to improve descriptions of ice forming processes. Cirrus conditions may seem an unusual choice for simulating heterogeneous ice nucleation, since the general view is that homogeneous freezing dominates (e.g., Heymsfield and Milosevich 1993). The impact of ice formation on cloud properties is generally thought to be much stronger in warmer clouds where supercooled water is more prevalent. These cirrus simulations only touched the surface of the possibilities that are provided by the capability to measure IN, to infer nucleation mechanisms, and to derive quantitative descriptions. There are many future applications that will lead to better understanding of the ways that ice nuclei function, their distributions in the atmosphere (e.g., to use as input to mesoscale forecast models), their sources, composition, etc. These will all ultimately benefit the forecasting of clouds and precipitation, and assessing the potential for climatic effects of aerosols through indirect mechanisms.
Predicting the initial formation of cloud ice requires improved
scientific understanding of the microphysical processes and appropriate
measurements of the initial thermodynamic, kinematic and physical/chemical
atmospheric environment. Ice nucleating aerosols comprise part
of the necessary information. There are techniques available
now to measure IN, but no single technique provides a complete
characterization. Most numerical cloud models use generic descriptions
of IN that are not be representative of specific conditions, and
they may not be representative in a general sense. Improvements
in predicting weather and climate must address the potential for
changes in aerosol loading and cloud-active properties, including
ice nucleation.
Acknowledgments
This research was supported by NASA grant NAG-2-924 and NSF grant ATM-9632917.
Bigg, E.K., 1957: A new technique for counting ice-forming nuclei in aerosols. Tellus, 9, 394-400.
Bigg, E.K., 1990: Measurement of concentrations of natural ice nuclei. Atmospheric Research, 25, 397-408.
Braham, R.R., W.A. Cooper, W.R. Cotton, R.D. Elliott, J.A. Flueck, J.M. Fritsch, A. Gagin, L.O. Grant, A.J. Heymsfield, G.E. Hill, G.A. Isaac, J.D. Marwitz, H.D. Orville, A.L. Rangno, B.A. Silverman and P.L. Smith, 1986: Precipitation enhancement -- a scientific challenge. Meteor. Monographs, 21, Amer. Meteor. Soc., Boston, 171pp.
Chen, Y., S.M. Kreidenweis, D.C. Rogers and P.J. DeMott, 1997: Single particle analyses of ice nucleating particles in the upper troposphere and lower stratosphere, Geophys. Res. Lett. (in review).
DeMott, P.J., D.C. Rogers, S.M. Kreidenweis, 1997a: The susceptibility of ice formation in upper tropospheric clouds to insoluble aerosol components, J. Geophys. Res. (in press).
DeMott, P.J., D.C. Rogers, S.M. Kreidenweis and Y. Chen, 1997b: The role of heterogeneous freezing nucleation in upper tropospheric clouds: Inferences from SUCCESS, Geophys. Res. Lett. (in review).
Heymsfield, A.J. and L.M. Miloshevich, 1993: Homogeneous ice nucleation and supercooled liquid water in orographic wave clouds, J. Atmos. Sci., 50, 2335-2353.
Kreidenweis, J.M., Y. Chen, D.C. Rogers and P.J. DeMott, 1997: Isolating and identifying atmospheric ice-nucleating aerosols: A new technique, Atmospheric Research (in review).
Langer, G., 1973: Evaluation of NCAR ice nucleus counter. Part I: Basic operation. J. Appl. Meteor., 12, 1000-1011.
Rogers, D.C., 1988: Development of a continuous flow thermal gradient diffusion chamber for ice nucleation studies, Atmospheric Research, 22, 149-181.
Rogers, D.C., 1994: Detecting ice nuclei with a continuous flow diffusion chamber - some exploratory tests of instrument response, J. Atmos. Ocean. Techn., 11, 1042-1047.
Rogers, D.C., P.J. DeMott, W.A. Cooper and R.M. Rasmussen, 1996: Ice formation in wave clouds - Comparison of aircraft observations with measurements of ice nuclei. 12th Intl. Conf. Clouds Precip., Zurich. 137-137.